Research Focus and Relevance
We combine molecular, preclinical animal models, and human intervention studies to explore the capacity of experiences and environments to leave their epigenetic marks on genes.
We are
interested in molecular mechanisms responsible for environmental
influences on CNS gene activity, development of behavior, and
psychiatric disorder.
Gene-environment interactions are recognized for their role in
shaping human behavior during early development, and epidemiological
research suggests that both an individual’s genes and the
environment underlie the pathophysiology of psychiatric disorders.
Molecular mechanisms mediating the interplay between genes
and the environment then are likely to have a significant role in
shaping such behavioral outcomes.
Epigenetic mechanisms, such as DNA methylation, are an
attractive molecular hypothesis to understand how environmental
signals are integrated at the genomic level to influence brain
development, CNS function, and behavioral development throughout the
lifespan. Research with animal models is invaluable for
understanding how the timing and duration of environmental variables
can interact to produce these changes in the brain and consequently
their relevance to behavior. Elucidation of the molecular processes
that regulate gene activity in response to environmental variables
will not only enhance the understanding of the biological basis of
behavior and disease, but promises hope for therapeutic
intervention.
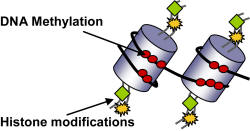
Schematic of epigenetic marking of the genome by DNA methylation and histone modifications, such as acetylation.
Our Approach
Projects in the lab use a variety of tissue types to understand gene-environment interactions. Some of the methods we use include bisulfite sequencing, methyl-specific PCR, chromatin immunoprecipitation, and quantitative real-time PCR for measuring gene expression.


Projects and Interests
Epigenetic and behavioral outcomes associated with early adversity
We have empirically demonstrated the long-term impact of even brief and mild negative experiences within the context of the caregiving environment on epigenetic mechanisms within several regions of the brain, as well as their heritable (F1 generation) and reversible nature. We have also discovered sexually-dimorphic epigenetic consequences of these negative experiences, and that some changes in DNA methylation, like the behavioral consequences of exposure to early adversity, do not manifest until later in life. Together, data from this body of work support the notion that epigenetic alterations within the CNS is one way that negative early-life experiences can produce long-term and even intergenerational consequences for neurobiology and behavior. Current projects in the lab are aimed at better understanding the causal relationship between epigenetic alterations and behavioral outcomes.

Undergraduates presenting their work on the amygdala and medial prefrontal cortex at research symposia hosted by The University of Delaware in 2011 and 2012.
Reversing the effects of early adversity in humans
We collaborate with clinical psychologists to study these same epigenetic marks in individuals, including young children, exposed to adversity. We study this in saliva. We also study the efficacy of intervention programs to change these marks and promote healthy physical and mental behavior.
To see our publications related to this work and other projects, click here.
All relevant data that support findings of studies are available on request.